Medicago completes cGMP qualification of its manufacturing facility
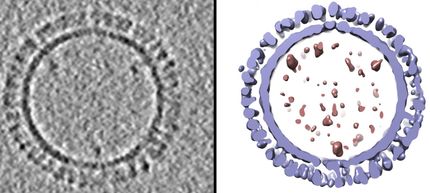
Medicago Inc. announced that it has successfully completed the current Good Manufacturing Practices ("cGMP") qualification of its manufacturing facility located in the Technology Park of Québec City, Canada. cGMP rules and regulations governing the development, manufacturing and control of pharmaceutical products apply to all stages of production, from early development to marketed products. This qualification will allow Medicago to produce clinical grade materials of H5N1 Avian Influenza virus-like particles ("VLP") vaccine candidates and other influenza vaccines.
"The qualification of our facility and quality control procedures to meet the cGMP and ISO standards are another important milestone in our program to advance our VLP pandemic influenza vaccine candidates," said Andy Sheldon, President and CEO of Medicago. "We are now in a position to take full advantage of our manufacturing capability and initiate the production of VLP vaccines this year to support our human clinical trials."
Medicago's facility is made up of 11,000 sq. ft. of Biosafety Level 2 greenhouse spaces for plant growth, as well as 3,000 sq. ft. of cGMP manufacturing suites for plant manipulation, product recovery and purification.
Most read news
Topics
Organizations
Other news from the department politics & laws

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.