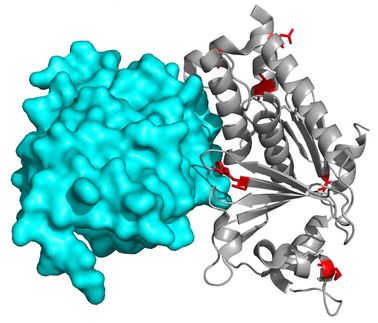
Crystallography reveals the 3D structure of ZP3 protein
Scientists at the Swedish medical university Karolinska Institutet have determined the first 3D structure of ZP3, a protein essential for the interaction between the mammalian egg coat and sperm. The findings, presented in Nature, gives a first glimpse into the molecular architecture of animal egg coats, with important implications for the future of human reproductive medicine and the possibility of developing novel contraceptives.
The beginning of every new life starts with fertilization, the most crucial step of which is the initial species-specific recognition between egg and sperm. The receptors for sperm, proteins ZP3 and ZP2, contain a common sequence that allows them to form a matrix of filaments, the so-called zona pellucida that completely surrounds the egg. The Protein Crystallography Unit at Karolinska Institutet, led by Dr. Luca Jovine, has now determined the structure of the most conserved part of this building block, the ZP-N domain.
“ZP3 was identified almost 30 years ago, but obtaining structural information on this key reproductive protein has been technically challenging due to its high heterogeneity”, says Luca Jovine.
The zona pellucida is essential for natural fertilization in mammals. The Karolinska Institutet researchers hope that X-ray crystallographic characterization of a region of ZP3 that is important for its ability to polymerize could help explaining cases of human infertility, as well as lead to the development of novel targeted, non-hormonal contraceptives. The research made on the ZP-N domain has also provided insights that extend beyond the field of reproduction. Among other things, an unexpected parallel has been uncovered with molecular features that are involved in speciation among invertebrates.
Moreover, ZP-N domains are also found in many other extracellular proteins that are unrelated to fertilization, but play important roles in human diseases such as non-syndromic deafness, renal and vascular disorders, and cancer. In the Nature paper, an example is discussed that shows how the structure of ZP-N can be used to understand the molecular basis of some of these disorders.
Original publication: Magnus Monné, Ling Han, Thomas Schwend, Sofia Burendahl, Luca Jovine; "Crystal structure of the ZP-N domain of ZP3 reveals the core fold of animal egg coats"; Nature 2008.
Most read news
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.