Novasep has Extended its Custom Chiral Separation Capabilities to Offer Optimized and Efficient Purification Solutions
Custom Chiral Separation Using Preparative Chromatography Eases and Speeds up Process Development; Rapidly and Efficiently Producing the Required Amount of Compounds for Early Development Phases While Being Cost Efficient at Industrial Scale
Novasep has recently added a state-of-the-art cGMP pilot scale continuous chromatography unit in its Chasse sur Rhône (France) facility. This ensures cost efficient separations of kilograms to tens of kilograms of pure enantiomer for clinical supplies.
Novasep operates six FDA-inspected facilities that manufacture APIs and advanced intermediates. Four of them integrate chiral separation services from preclinical to commercial supply. Novasep now operates ten pilot and commercial scale HPLC units, eight pilot and commercial scale Varicol(R) systems (continuous chromatography systems) for producing batches up to 1 metric ton with a total capacity of more than 150 metric tons per annum.
This investment expands Novasep's chiral separation capabilities. The fully operational plant includes a Varicol(R) 8-80 unit, using 5 to 8 columns of 80mm i.d. and incorporates the latest PAT-compliant advanced control technology.
Most read news
Topics
Organizations
Other news from the department manufacturing
These products might interest you

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Chromatography
Chromatography enables us to separate, identify and thus understand complex substances. Whether in the food industry, pharmaceutical research or environmental analysis - chromatography opens up a treasure trove of information about the composition and quality of our samples. Discover the fascinating world of chromatography!

Topic World Chromatography
Chromatography enables us to separate, identify and thus understand complex substances. Whether in the food industry, pharmaceutical research or environmental analysis - chromatography opens up a treasure trove of information about the composition and quality of our samples. Discover the fascinating world of chromatography!
Last viewed contents
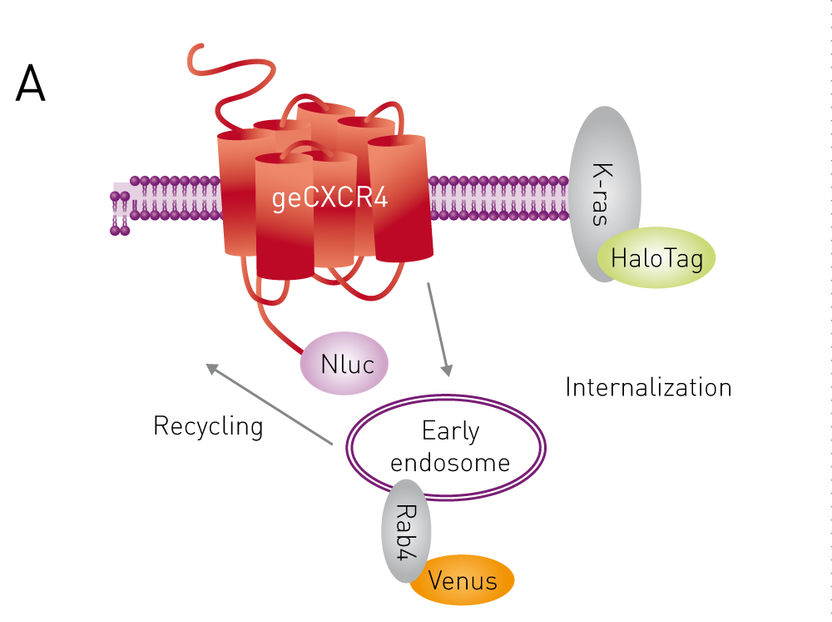
Nanobret ™ -Based Assay for Internalization of cxcr4 - Read how GPCRs are studied at endogenous expression levels by employing CRISPR/Cas9.
New material for detecting photons captures more quantum information

Detecting bacteria with fluorescent nanosensors - Luminous carbon nanotubes detect pathogens – and are quick and easy to use
Agilent Technologies introduces industry's first HPLC-chip/MS system for proteomics