New synthetic route to oxindoles
Peter Kündig and colleagues from Geneva University, Switzerland and Stephen Marsden and co-workers at the University of Leeds, in the UK, have developed an asymmetric Pd/N-heterocyclic carbene (NHC) - catalysed intramolecular -Arylation of amide enolates, containing heteroatom sustituents. This catalytic reaction produced optically active oxindole derivatives.
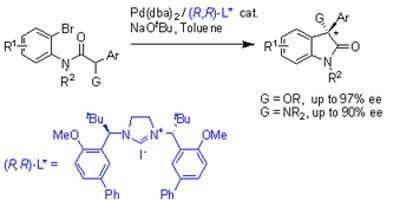
This synthetic route could be useful in the synthesis of biologically active chiral oxindoles. This work is significant as oxindoles constitute many natural products and biologically active compounds that could be developed into drugs.
Optically active oxindoles exhibit a large variety of pharmacological activity, particularly affecting neurological functions, which has led to intense activity in the asymmetric synthetic routes to prepare these compounds.
The next step is 'understanding why these particular ligands work so much better than previously tested ligands belonging to the same family (NHC's) and applying the method to more complex bioactive molecules,' said Kündig.
Original publication: E. Peter Kündig et al., Chem. Commun., 2008.
Most read news
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.