How Molecules Break and Form Bonds
A crucial step toward truly understanding chemical processes
Researchers at European XFEL in Germany have tracked in real time the movement of individual atoms during a chemical reaction in the gas phase. Using extremely short X-ray flashes, they were able to observe the formation of an iodine molecule (I₂) after irradiating diiodomethane (CH₂I₂) molecules by infrared light, which involves breaking two bonds and forming a new one. At the same time, they were able to distinguish this reaction from two other reaction pathways, namely the separation of a single iodine atom from the diiodomethane, or the excitation of bending vibrations in the bound molecule. The results provide new insights into fundamental reaction mechanisms that have so far been very difficult to distinguish experimentally.

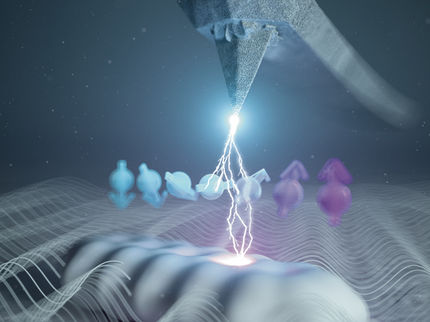
Diiodomethane irradiated with infrared light can undergo several different reactions. Intense X-ray pulses of European XFEL and a reaction microscope of the SQS instrument were used to characterize three major reaction pathways.
Tobias Wüstefeld, © European XFEL
So-called elimination reactions in which small molecules are formed from a larger molecule are central to many chemical processes—from atmospheric chemistry to catalyst research. However, the detailed mechanism of many reactions, in which several atoms break and re-form their bonds, often remains obscure. The reason: The processes take place in incredibly short times—in femtoseconds, or a few millionths of a billionth of a second.
An innovative experimental approach was now used at the SQS instrument at European XFEL to visualize such reaction dynamics. The researchers irradiated diiodomethane molecules with ultrashort infrared laser pulses, which triggered the molecular reactions. Femtoseconds later, intense X-ray flashes shattered the molecules, causing their atomic components to fly apart in a "Coulomb explosion." The trajectories and velocities of the ions were then recorded by a detection device called the COLTRIMS reaction microscope (COLd Target Recoil Ion Momentum Spectroscopy)—one of the detection instruments at the SQS experimental station that is made available to users.
"Using this method, we were able to precisely track how the iodine atoms assemble while the methylene group is cleaved off," explains Artem Rudenko from Kansas State University, USA, the principal investigator of the experiment. The analysis revealed that both synchronous and asynchronous mechanisms contribute to the formation of the iodine molecule—a result that was supported by theoretical calculations.
Remarkably, "Although this reaction pathway only accounts for about ten percent of the resulting products, we were able to clearly distinguish it from the other competing reactions," explains Rebecca Boll from the European XFEL's SQS (Small Quantum Systems) instrument in Schenefeld near Hamburg. This was made possible by the precise selection of specific ion fragmentation channels and their time-resolved analysis.
Furthermore, the researchers were able to track the vibrational motion of the newly formed iodine molecule. "Now, we can more directly observe how an isolated molecule breaks and forms bonds during a chemical reaction—in real time and with atomic precision," says Xiang Li, the first author of the publication and a scientist at the SLAC National Accelerator Laboratory in the United States. This is a crucial step toward truly understanding chemical processes. These observations not only provide a detailed picture of reaction mechanisms but also open up new avenues for investigating more complex chemical processes.
In the future, these techniques will be extended to even larger molecules and more complex reactions. Thanks to planned technical improvements to the European XFEL X-ray laser, even faster and more detailed insights into the world of ultrafast molecular dynamics can be gained in the future.
Original publication
Xiang Li, Rebecca Boll, Patricia Vindel-Zandbergen, Jesús González-Vázquez, Daniel E. Rivas, Surjendu Bhattacharyya, Kurtis Borne, Keyu Chen, ... Sergey Usenko, Anbu Selvam Venkatachalam, Enliang Wang, James P. Cryan, Michael Meyer, Till Jahnke, Phay J. Ho, Daniel Rolles, Artem Rudenko; "Imaging a light-induced molecular elimination reaction with an X-ray free-electron laser"; Nature Communications, Volume 16, 2025-7-30
Most read news
Original publication
Xiang Li, Rebecca Boll, Patricia Vindel-Zandbergen, Jesús González-Vázquez, Daniel E. Rivas, Surjendu Bhattacharyya, Kurtis Borne, Keyu Chen, ... Sergey Usenko, Anbu Selvam Venkatachalam, Enliang Wang, James P. Cryan, Michael Meyer, Till Jahnke, Phay J. Ho, Daniel Rolles, Artem Rudenko; "Imaging a light-induced molecular elimination reaction with an X-ray free-electron laser"; Nature Communications, Volume 16, 2025-7-30
Organizations
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Screening for Co-Crystals with a Solubility-based Approach with Crystal16 - Discover a systematic approach for screening co-crystals

AI technique 'decodes' microscope images, overcoming fundamental limit - “We’ve given a proof-of-concept and shown how to use AI to significantly improve AFM images, but this work is only the beginning”
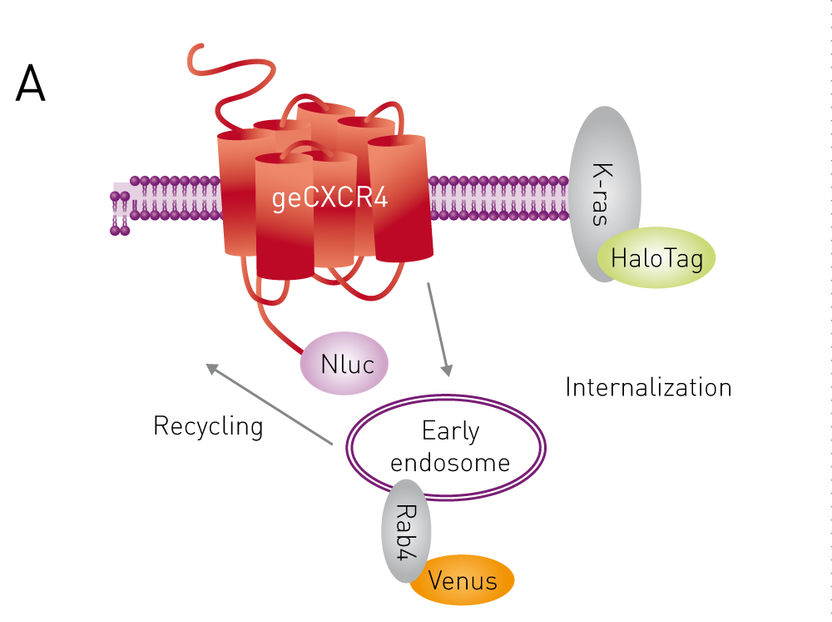
Nanobret ™ -Based Assay for Internalization of cxcr4 - Read how GPCRs are studied at endogenous expression levels by employing CRISPR/Cas9.