Energy hijacking pathway found within photosynthesis
An unexpected source of inefficiency within a photosynthetic enzyme has been identified by scientists. The issue also adversely affects the performance of devices which are used to model artificial photosynthesis - a biomimicry process which is central to efforts to generate sustainable fuel by converting sunlight into chemical energy.
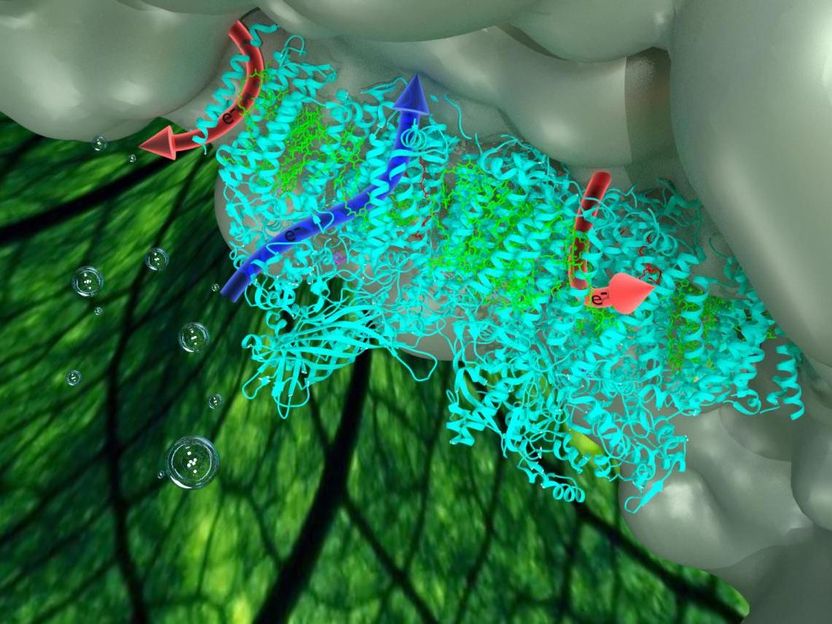
Competing electron transfer pathways are found within Photosystem II using an advanced technique known as protein-film photoelectrochemistry. In one pathway, electrons are extracted from water by the enzyme. In the other, electrons are hijacked, generating reactive radical species.
Jenny Zhang
The study, carried out by a University of Cambridge team, reveals hitherto-unknown properties within Photosystem II, the enzyme which kick-starts photosynthesis in plants by extracting electrons from water using sunlight.
Specifically, the group were able to analyse and describe a "pathway" by which light energy needed to extract the electrons was being hijacked by oxygen, which is a by-product of the process.
They were able to bypass this competing pathway temporarily by removing the electron-stealing oxygen. By further embedding the Photosystem 2 in a suitable electron conducting matrix, they were able to boost the efficiency of a semi-artificial photosynthetic system. Such systems are typically created by wiring together parts of the natural photosynthetic machinery found in plants with synthetic materials.
This type of hybrid structure serves as a blueprint for wholly-artificial systems which scientists are trying to develop. The aim is to use these to imitate and surpass natural photosynthesis, so that sunlight can be used to efficiently "split" water into its component parts, hydrogen and oxygen, enabling the hydrogen to be extracted as fuel.
The research was carried out by a team from the Reisner Lab in the Department of Chemistry at the University of Cambridge. The Lab specialises in developing sustainable fuel-producing systems, based on artificial photosynthesis.
Dr Jenny Zhang, a Research Associate at St John's College, University of Cambridge, and the paper's first author, said: "Our study used a technique called protein-film photoelectrochemistry, which allows us to see the process by which charges in the enzyme are transferred to the electrode upon light irradiation."
"This helps us to know more about the enzyme. We were able to see that there was a pathway by which electrons were being diverted away from the electrode, reducing its efficiency, and then address that. It is likely that the same competing pathway problem will crop up in other synthetic solar fuel devices that we try to manufacture, so the importance of this insight extends far beyond Photosystem II."
"In addition, it is possible that this pathway exists in nature for all photosystem-like enzymes, contributing towards lowered efficiency within photosynthetic organisms."
In nature, Photosystem II performs part of the "water-splitting" reaction that researchers are keen to imitate, efficiently and at low cost, so that renewably-produced hydrogen can be extracted as fuel. This would be a starting material for making synthetic petrol and fertiliser.
Water-splitting is actually a two-part process, in which water is first broken down into oxygen and protons, and the protons are then combined to make hydrogen. Crucially, Photosystem II uses energy from sunlight to carry out the first phase - water oxidation. It achieves this at high rates, using only Earth-abundant materials. This is something that scientists struggle to mimic.
As the water is oxidised, a flow of electrons are released across the enzyme, which are used in the second stage to stick the protons together. In hybrid systems, these are first gathered by an electrode, but the wiring between the enzymes and the electrodes has been disappointingly inefficient to date, making it difficult for humans to control and exploit the enzymes in useful ways.
The new study offers a major clue about why those efforts have been so underwhelming. Alongside the main route by which electrons are shuttled across the enzyme, a second - and counterproductive - pathway exists. This only became visible in the protein-film electrochemistry experiments.
The second pathway plunders electrons from any available source, notably the electrode. It draws them towards oxygen molecules that have already been created within the enzyme, as part of the oxidation process. In effect, it "steals" energy, short-circuiting and reducing the system's overall performance.
As well as unfavourable, the effect is also potentially destructive. As the electrons are taken up by the molecular oxygen, they form a reactive compound, hydrogen peroxide, which damages the enzyme and the system that contains it. Effectively, the pathway "self-harms" the system.
While in living organisms the formation of these damaging species is likely to be mitigated by other natural processes, if humans are to manufacture anything like Photosystem II in the future to create artificial photosynthetic devices, they will have to find a way to do the same thing.
The group believe, however, that the prospects for doing so are relatively good. Zhang explored strategies for controlling the competing pathway in the new study. She demonstrated that the enzymes' performance is enhanced by the pathway's removal, and that this makes the task of measuring the enzyme's physical properties easier, which is important to its value as a blueprint system.
"We have lots of ideas about how to resolve the competing pathways problem," she added. "It may involve making careful choices about the materials with which we make the electrode, or coating the sides of the enzyme so that energy cannot be stolen."

























































