Loss of essential protein in the choroid plexus epithelium linked to hydrocephalus
A team led by researchers at St. Jude Children's Research Hospital report that mice lacking the protein Alix develop hydrocephalus or "water on the brain." Alix ensures that epithelial cells of the choroid plexus are oriented correctly with respect to one another to prevent compromise of the epithelial barrier. The research appears online today in the scientific journal Nature Communications.
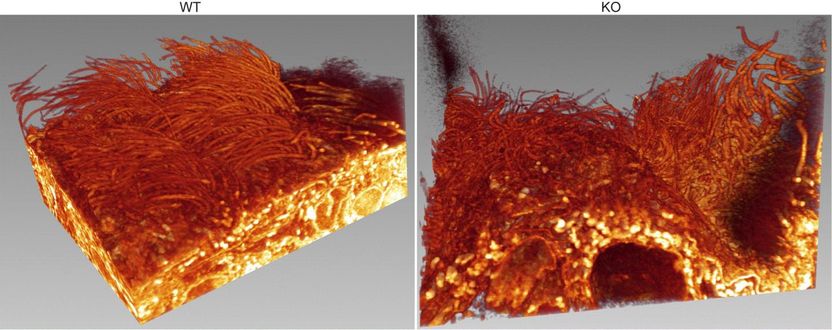
Loss of Alix (right) also results in defective orientation of motile cilia in the ependyma -- the protective epithelial barrier between brain and cerebrospinal fluid.
"We have successfully developed the first mouse model for Alix that allows us to study the consequences of Alix loss of function in vivo. We were intrigued by the occurrence of hydrocephalus in the brains of these mice and began to investigate the structures within the brain that might be involved - the choroid plexus and the ciliated ependyma," said corresponding author Alessandra d'Azzo, Ph.D., who holds the Jewelers For Children Endowed Chair in Genetics and Gene Therapy at St. Jude. "Our study unravels the central role that Alix plays not only in preserving the general architecture of the epithelium and the epithelial barrier but also how this protein contributes to the maintenance of brain homeostasis."
The researchers painstakingly examined how Alix exercises these functions to promote the correct assembly and positioning of specialized intercellular junctions between epithelial cells called the tight junctions. By interacting with the actin cytoskeleton, underneath the cell outer membrane, and other structural proteins of the tight junctions, Alix ensures the formation of the actomyosin-tight junction complex at a specific position between adjacent cells. Alix therefore functions as a "molecular bridge" that also determines the polarity of individual epithelial cells, namely the way cells are oriented with respect to one another within the epithelial cell layer.
"We showed that the loss of Alix causes striking defects in actomyosin assembly and tight junction formation. These changes are so fundamental that they lead to prominent alterations in cell shape and loss of cell polarity, alterations that ultimately affect the epithelial barrier," explained d'Azzo.
The epithelial barrier in the choroid plexus of the brain is the blood-cerebrospinal fluid interface. It is a crucial control point for the movement of essential ions, molecules and other metabolites, some of which help keep the production of cerebrospinal fluid finely balanced. On the one hand, cerebrospinal fluid serves as a protective barrier against mechanical damage and disease. However, excessive accumulation can lead to adverse health conditions such as hydrocephalus, as observed in this investigation.
Yvan Campos, associate scientist in the St. Jude Department of Genetics and the paper's first author, was instrumental in defining Alix's precise role in the brain's choroid plexus and linking that role to disease. This was extremely challenging, since many disparate functions have been attributed to Alix in different cell types. "We made extensive use of state-of-the art, high-resolution imaging techniques to visualize the impact of Alix deficiency on the overall organization of the choroid plexus epithelium," said Campos.
It was through the analysis of these high-resolution images that the team made the important observation that loss of Alix leads to an increased number of cells caught in the act of being discharged from the epithelial layer. The process, called "cell extrusion," is the way a new cell replaces an existing one in the epithelium by "pushing up" the cell to be discarded. "It is an important physiological mechanism for the ordered removal of cells from the epithelial layer, but when it is out of control has profoundly damaging effects," said Campos. Unrestrained epithelial cell extrusion has been linked to tumor cell invasion and metastasis in other studies.
Looking ahead, this newly generated mouse model offers far-reaching scope to study the molecular bases of hydrocephalus, a fairly common clinical condition in humans, where the causes are often unknown. In addition, the combination of defective epithelial barrier and abnormal epithelial cell extrusion observed in this mouse model may be further exploited to study the role of Alix in cancer.
Most read news
Organizations
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.





















































