Cytiva and Pall Corporation investing 1.5 billion USD over two years to meet growing demand for biotechnology solutions
Major investments are expanding manufacturing capacity for life sciences products at 13 Cytiva and Pall Corporation sites helping to meet customer demand.
An ongoing strategic growth plan from Cytiva and Pall Corporation, part of the Danaher Corporation, will expand manufacturing capacity and services across geographies for global life sciences customers.
The investment, already underway, includes new sites, expansion at existing factories, and is additional to previously announced investments. It follows five acquisitions made by the companies so far this year.
Emmanuel Ligner, Danaher Group Executive, says: “Our customers tell us they need access to manufacturing agility, a robust global supply chain and more regional options. This investment further fuels our expansion program so we can rapidly meet the current and future needs of our customers and ultimately, their patients.”
Cytiva and Pall Corporation’s capacity expansion will increase the manufacture of key products used to make biologic medicines.
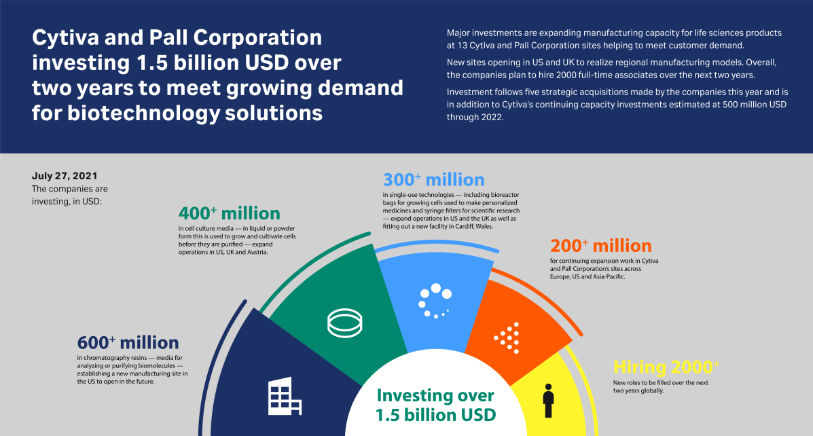
The companies are investing:
- 600+ million USD in chromatography resins – media for analyzing or purifying biomolecules – establishing a new manufacturing site in the US.
- 400+ million USD in cell culture media – in liquid or powder form this is used to grow and cultivate cells before they are purified– expand operations in the US, UK, and Austria.
- 300+ million USD in single-use technologies – including bioreactor bags for growing cells used to make personalized medicines and syringe filters for scientific research – expand operations in the US and the UK, as well as fitting out a new facility in Cardiff, Wales.
- 200+ million USD for continuing expansion work in Cytiva and Pall Corporation’s sites across China and the rest of the Asia-Pacific region, Europe, and the US.
This investment also addresses some of the key challenges highlighted in the Global Biopharma Resilience Index, conducted by Longitude, a Financial Times company, and published by Cytiva in March 2021. These include hiring and training talent, R&D collaboration, supply chain resilience, manufacturing models, as well as government policy and regulation.
Rapid expansion program
Funds are being allocated to accelerate capacity expansion plans already underway at 13 Pall Corporation and Cytiva sites making biotechnology products for COVID-19 and other critical programs.
For example, production of cell culture media at Cytiva’s site in Logan, Utah is planned to double in 2023. This will be done by converting 25 000 m2 of land into new manufacturing lines, distribution hubs, and clean rooms as well as creating 250 new jobs locally. Combined with the acquisition of Intermountain Life Sciences, a manufacturer of liquid cell culture media and buffers, customers being supplied out of Logan will see shorter lead times in delivery of those products.
Additionally, investments at Pall sites in Newquay, UK, Pensacola, FL, and San Diego, CA have increased capacity to make and deliver membrane and filter products.
The 1.5 billion USD investment follows Cytiva’s announcement in 2020 to spend 500 million USD building capacity. The investment has already funded a new manufacturing facility in Shrewsbury, MA and a new cleanroom in Westborough, MA in the US. A new cell and gene therapy manufacturing site and Center of Excellence is scheduled to open in Switzerland in 2022.
As part of today’s investment, Pall Corporation will expand single-use manufacturing operations in China at the site of recently acquired Austar. Plans are also underway to expand Cytiva’s partnership with Wego in China to build more single-use technology manufacturing capacity for the region. Both companies plan for further regional investment in the future.
New facilities
A new 11 000 m2 facility in Cardiff, Wales, is being fitted out to manufacture single-use bioprocessing equipment including jumper tubing assemblies, cell bags, and ÄKTA flow kits. The first products are expected to be manufactured at this site before the end of 2021. A local hiring drive has begun to fill 250 new jobs over the next two years, primarily in manufacturing, as well as warehouse operators, material handlers, and R&D scientists.
The vision for the new US facility is to replicate and complement Cytiva’s leading resin manufacturing capabilities at its site in Uppsala, Sweden. The location of the new site will be announced at a later date. Cytiva plans to hire 400 people in the US and Sweden over the next two years to facilitate expansion plans.
Investing in talent
Overall, the companies are planning to hire 2000 people over the next two years to support growth. This is additional to the 2000 new associates hired by Cytiva and Pall Corporation over the last year.
The approach considers the well-established skills gap in the biotechnology industry. For example, training for manufacturing roles will involve intensive on-site courses. Onboarding for highly technical roles will leverage Cytiva’s FastTrak centers and online training designed in partnership with the National Institute for Bioprocessing Research and Training (NIBRT) in Dublin, Ireland.





























































