How cells move
It's a known fact that cells can move around the body, but how they do it has been unknown - until now. Researcher in Infection Medicine Pontus Nordenfelt at Lund University in Sweden has managed to describe and visualize cell migration on a molecular level. In time, this could become significant in the treatment of infectious diseases, inflammation, cancer, etc. where cell migration plays an important role.
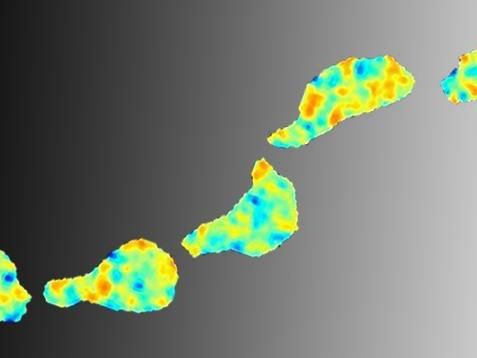
A cell on the move. The process of migration shown in images taken with 15 second intervals. The colors show the force needed to migrate -- red representing the most force.
Pontus Nordenfelt, Lund University
In his studies, Pontus Nordenfelt used T cells as models. The T cells are part of the immune system and must therefore be able to reach tissue which has been exposed to, for instance, a bacterial invasion.
To be able to move, the cell must attach itself to a surface and use its front to push to exert the force it needs. Meanwhile, the rear part of the cell must let go from the surface, allowing it to "roll" forward, so to speak.
"When moving, the cell converts chemical energy into mechanical force. This force can now be measured using the microscope tool I developed", says Pontus Nordenfelt, an engineer from the start, whose work benefited greatly from having this background.
Cell migration is enabled by three molecules working together. Integrins are molecules located on the cell surface that are able to attach themselves to other surfaces. Actin are small building blocks on the inside of the cell membrane, and collectively form the skeleton of the cell. Finally, the "adaptor" is a protein that links integrin and actin together.
"Once the adaptor has connected the integrins and actin, the mechanical force from actin gives the 'green light'. The integrin molecule then binds itself to a nearby partner surface, through which the cell can move slightly forward. Once the cell's migration is complete, the integrin and actin molecules separate from each other in this part of the cell, while another part of the cell becomes active", explains Pontus Nordenfelt.
The key to cell migration is that the integrin molecules become active in the appropriate part of the cell, and how this works has so far been unclear.
The notion that cell migration would be based on activation of integrins through force from actin was suggested several years ago by prominent researcher Timothy Springer at Harvard, where Pontus Nordenfelt spent three years examining how integrins become active. Among other things, the work resulted in new microscopy techniques that have been used to test this hypothesis of how cell migration works.
The technique is based on fluorescent biosensors that are inserted into the cell, showing force changes in integrins through variations in colour. This allows you to see the process of cell migration through colour images, and measure the mechanical force acting on the integrins. This technique could enable the development of more targeted drugs to strengthen the body's immune system against infections, or prevent tumours from metastasising, etc.
As a researcher in Infection Medicine, Pontus Nordenfelt became interested in integrins, as this is a group of proteins used by the immune cells, which led to his studies of cell migration. With the help of his new tool, he now wants to continue with the study of phagocytes - immune cells that "eat" invading bacteria.
"The fluorescent biosensors enable measuring when and how different bacteria are ingested; perhaps it's also possible to create variants of the sensors that measure the opposite, that is, when bacteria avoid being eaten and instead manage to invade the body", he says.
Original publication
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Fluorescence microscopy
Fluorescence microscopy has revolutionized life sciences, biotechnology and pharmaceuticals. With its ability to visualize specific molecules and structures in cells and tissues through fluorescent markers, it offers unique insights at the molecular and cellular level. With its high sensitivity and resolution, fluorescence microscopy facilitates the understanding of complex biological processes and drives innovation in therapy and diagnostics.
Topic world Fluorescence microscopy
Fluorescence microscopy has revolutionized life sciences, biotechnology and pharmaceuticals. With its ability to visualize specific molecules and structures in cells and tissues through fluorescent markers, it offers unique insights at the molecular and cellular level. With its high sensitivity and resolution, fluorescence microscopy facilitates the understanding of complex biological processes and drives innovation in therapy and diagnostics.