Bio-marker set forms the basis for new blood test to detect colorectal cancer
colorectal cancer is the third most common form of cancer globally and the second most common cause of cancer deaths. The chance of a cure is high if the cancer is detected early enough, but early detection is not a given. Researchers from VIB and KU Leuven - together with various European oncology centers, including UZ Leuven - have identified bio-markers that can be incorporated in a new diagnostic test. This should make it possible to detect colorectal cancer in an early stage using a simple blood test.
Max Mazzone (VIB/KU Leuven): “This research demonstrates how important it is to gain a thorough understanding of the role of our immune system in cancer. In this case, this knowledge will hopefully result in a new, more sensitive test to detect colorectal cancer at an early stage, so that more patients can be cured. I hope that we can soon find an industrial partner to help us achieve the following step, which is the development of the test.”
Population screening
There are no global screening guidelines, but because early detection is so important, there are a number of national initiatives to screen the population. For example, in Flanders, the population group between the ages of 56 and 74 years is invited to undergo testing via the “immunological Fecal Occult Blood test” (iFOB), which detects blood in the stools. If this test is positive, a colonoscopy needs to be performed to confirm the presence of premalignant polyps or cancer.
Even though the iFOB test is the best test available, sensitivity is suboptimal. In other words the available test doesn’t detect all colon cancers. There is a need for a test that offers greater certainty and that can detect bowel cancer at an early stage and at the same time reaches the whole population. If this can be achieved with a blood test, this might lower the reluctance seen in patients towards the stool test.
Our immune system responds to cancer
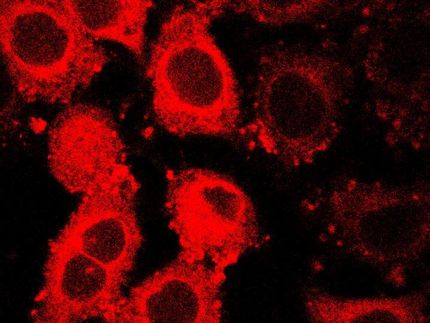
If we are affected by cancer, our immune system responds to this and tries to remove the cancer cells from our body. A specific role in this process is assigned to a specific type of white blood cell: the peripheral blood monocyte. From the moment that colorectal cancer cells are present in the body, the peripheral blood monocytes respond to the substances secreted by the cancer cells.
Set of bio-markers identified
For the identification of the genes involved in this process, Hans Prenen (UZ Leuven) and his colleagues from oncology centers in Brussels, Heidelberg and Rome collected samples from patients. This allowed the researchers led by Max Mazzone (VIB/KU Leuven) to identify 43 relevant genes.
The challenge facing the investigators now is to develop a test using a minimal set of bio-markers. They are looking for an industrial partner for the development of the test.
Original publication
Hamm, Prenen, Van Delm et al.; "Tumour-Educated Circulating Monocytes are Powerfule Candidate Biomarkers for Diagnosis and Disease Follow-up of Colorectal Cancer."; Gut 2015
Most read news
Original publication
Hamm, Prenen, Van Delm et al.; "Tumour-Educated Circulating Monocytes are Powerfule Candidate Biomarkers for Diagnosis and Disease Follow-up of Colorectal Cancer."; Gut 2015
Organizations
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.