Data to aid production and storage of 'fascinating' medication
Amantadine hydrochloride may be the most common medication you've never heard of. This compound has been around for decades as the basis for antiviral and other medications, from flu therapy to treatments for brain disorders such as Parkinson's disease and the fatigue associated with multiple sclerosis.
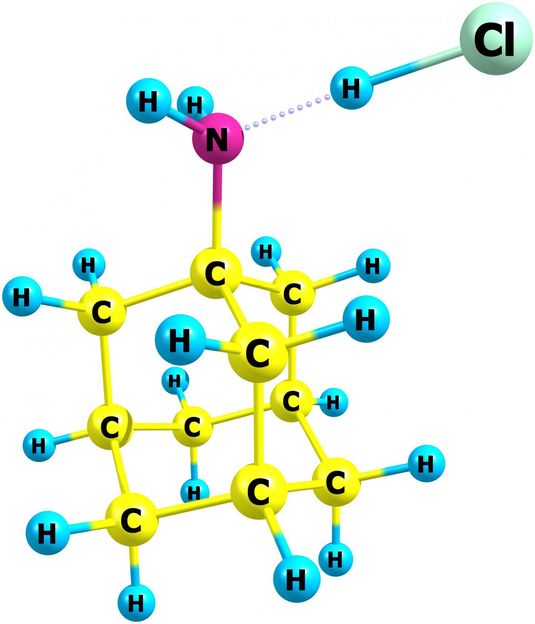
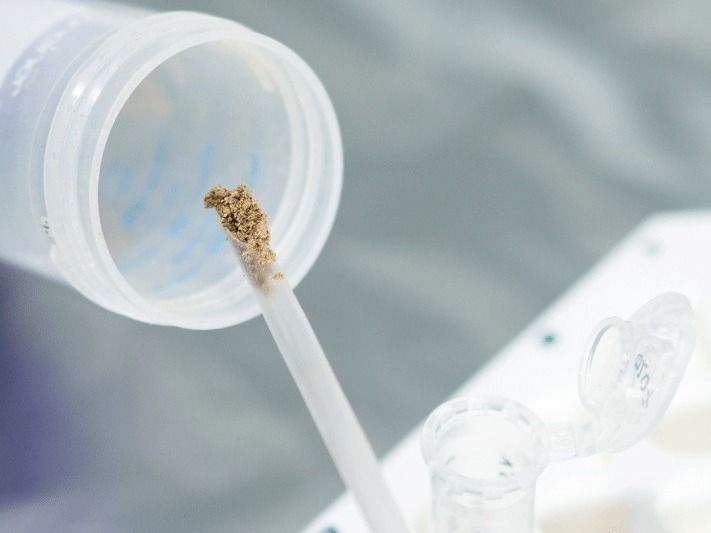
NIST chemists have published the first data on the thermodynamic properties of amantadine hydrochloride, used for many years as the active pharmaceutical ingredient for antiviral and anti-parkinsonian medications. The new information can help optimize production and storage conditions of this important compound. Its structure in the gas phase was obtained by quantum chemistry methods. In the molecular structure, C is carbon, H is hydrogen, N is nitrogen, and Cl is chlorine.
Bazyleva/NIST
And yet, this compound has long been a bit of an enigma because of missing information on its properties. Now, chemists at the National Institute of Standards and Technology (NIST) and collaborators have published the very first data on this important chemical's thermodynamic properties, including data on how it responds to heat and changes from a solid into a gas.
Such data are valuable to the chemical and pharmaceutical industries for getting the highest production yields and shelf life for the medication.
"Our research results are not directly related to the medical application of this multifunctional drug, although I am really fascinated by the range of its pharmacological activity," NIST research chemist Ala Bazyleva said.
"We studied its thermodynamic properties and decomposition," Bazyleva said. "It is surprising, given the long history of amantadine-based drugs, that there is almost no information like this in the literature for many of them. Chemical engineers often have to rely on estimates and predictions based on similar compounds. Collating this information and developing these types of recommendations is at the core of what our group at NIST does."
Amantadine hydrochloride belongs to a diamondoid class, a family of compounds whose structure is based on a cage of carbon atoms similar to diamond. Amantadine has a single carbon cage with a nitrogen atom attached on one side. Nonmedical studies have focused on the solid form of amantadine hydrochloride because it was expected to form disordered, or plastic, crystals, as many diamondoids do. Turns out, amantadine hydrochloride does not.
Bazyleva began studying amantadine hydrochloride years ago while in Belarus working on her doctoral dissertation, and continued the effort during her postdoctoral studies in Germany and Canada. But progress was slow, partly because adamantine hydrochloride changes from a solid directly into a gas (a process called sublimation) and simultaneously falls apart, or decomposes. She needed a model explaining this complex process, one that incorporates detailed, high-level calculations of quantum chemistry. She finally got access to this computational capability after she began working with the Thermodynamics Research Center (TRC) Group at NIST in Boulder several years ago.
"NIST was fundamental in facilitating the modeling component," Bazyleva said. "In particular, the unique combination of facilities, software and expertise in quantum chemical computations allowed us to apply high-level calculations to get insight into the structure and stability of the drug in the gas phase."
While the compound behaves like it is ionic (composed of positively and negatively charged pieces, though neutral overall) in the solid crystal form and when dissolved in a liquid, quantum chemistry calculations revealed that it decomposes into two neutral compounds in the gas phase.
Original publication
Most read news
Original publication
Ala Bazyleva, Andrey V. Blokhin, Dzmitry H. Zaitsau, Gennady J. Kabo, Eugene Paulechka, Andrei Kazakov, and John M. Shaw; "Thermodynamics of the Antiviral and Antiparkinsonian Drug Amantadine Hydrochloride: Condensed State Properties and Decomposition"; Journal of Chemical & Engineering Data; 2017
Topics
Organizations
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Zoom in to watch DNA code being read
New technology to study virus infections - "It may be possible to rapidly address emerging pandemics in the future by tailoring our tool to recognize novel viral strains"
New test opens path for better 2-D catalysts
Nanobiosensor Developed for Detecting SARS-CoV-2 - New sensor technology breakthrough against the pandemic
DNA from extinct humans discovered in cave sediments - New method retrieve hominin DNA from cave sediments – even in the absence of skeletal remains

Researchers have succeeded for the first time in analyzing nanovesicles and proteins in parallel - This is important to determine the quality of a sample and to clearly attribute effects to the transport vesicles

Physicists create giant trilobite molecules - Results are important to understand the chemical binding mechanisms of them, which is distinct from all other chemical bonds

New resource for optical chips

Electron bubbles modelled from X-ray laser data - An international team of scientists uncovers a groundbreaking model for the effects of radiation in water systems

AIMe - A Standard for Artificial Intelligence in Biomedicine
Initiative taps scientists to create atlas of cells in human spinal cord




















































