New tool to unravel the mysteries of metastasis
Researchers at the UNC School of Medicine have devised a new biochemical technique that will allow them and other scientists to delve much deeper than ever before into the specific cellular circuitry that keeps us healthy or causes disease.
The method – developed in the lab of Klaus Hahn, PhD, and described in the journal Nature Chemical Biology – helps researchers study how specific proteins called kinases interact to trigger a specific cellular behavior, such as how a cell moves. These kinase interactions are extraordinarily complex, and their interactions remain largely unknown. But researchers do know that kinases are crucial operators in disease.
"I dare you to find a disease in which kinases are not involved," said Hahn, senior author of the study and the Thurman Distinguished Professor of Pharmacology. "These kinase processes have been very difficult to fully understand, but we all know they're very important."
For years, scientists have been able to tweak a kinase to see what would happen – such as causing cell death or cell movement or cellular signaling. But these experiments can only scratch the surface when it comes to understanding the cascade of kinase interactions that lead to a cellular behavior. Nor have these experiments been able to show the timing of rapid events. That's important, Hahn said, because when a protein is activated has a lot to do with how the cell will respond. Drug developers haven't been able to take this into account, which is likely one reason why some drugs that target proteins don't work as well as scientists had hoped.
"Imagine you're an electrician looking at a circuit board, and all you can do is plug something in and watch all the circuits light up, but you have no idea how the board really works," Hahn said. "What you'd like to do is put a probe on one component, turn it on, and see what immediately happens to the circuit components next to that one component."
If you could do this with all the circuit components, then this would allow you to learn how the circuitry is built.
"We are now doing this in live cells and seeing what happens," said Hahn, a member of the UNC Lineberger Comprehensive Cancer Center. "Kinases are the circuit components. And we can now activate just one kinase and study how it interacts with just one other molecule in real time."
These kinase circuits are critical for cellular activities, such as metabolism, signaling, protein regulation, movement, enzyme secretion, and many others. All kinases have nuanced differences but all of them share one little part that researchers call a domain.
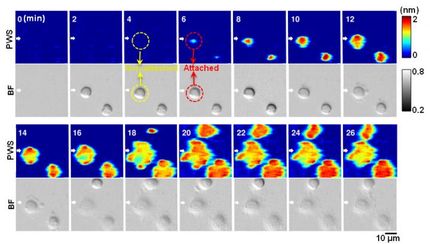
Hahn's team, led in the lab by postdoctoral fellow Andrei Karginov, PhD, studied the sarcoma kinase (Src) and figured out a way to use that part to attach an artificial protein to render Src inactive. That artificial protein had a binding site. When Karginov added a drug analog to the solution in which the cell lived, the drug analog bound to that site, causing the kinase to reactivate. Karginov could activate the kinase to see how the circuits lit up – how the cell responded at any given time during the cell's transition from a stationary cell to a moving, metastatic cell.
They could see the reaction in real time, so they knew that what they did caused the cell to react. Other methods struggle with this. Genetically manipulating a cell, for instance, takes too much time, Hahn said. Before you can see the results of the experiment, other proteins compensate for the kinase that was shut down.
Hahn's technique got around that problem, which allowed his lab to take their work one step further.
Karginov developed a two-component system. In this new system, adding the drug caused the activated kinase to interact only with molecules that contain a second engineered protein. Not only could Karginov turn on the kinase at an exact time; he could now tell the kinase exactly which circuit component to interact with.
They found that when Src was linked only to the kinase FAK, the cell's shape changed; it extended huge arms, or protrusions, but the cell didn't create new protrusions. When Src was linked with only the kinase CAS, the cell added new protrusions and the cell's adhesion ability improved. These are the behaviors that cancer cells need to move. In essence, Hahn's lab figured out a way to pinpoint precise mechanisms underlying metastasis.
"What this paper really does is show how all of this can be done to any kinase you want," Hahn said. "Our lab is interested in metastasis. But our hope is that our tool goes well beyond our narrow field of study. You just have to ask yourself, 'how important are kinases to disease?' And the answer is they're very important; they are everywhere."