Toxicity testing on the placenta and embryo
Detection substances that are harmful to the embryo at an early stage of drug development
Researchers at ETH Zurich have developed a cell culture test to detect substances that are directly or indirectly harmful to embryos. Based on an existing test used for developing new drugs and chemicals, the augmented version is designed to help reduce the number of animal experiments.
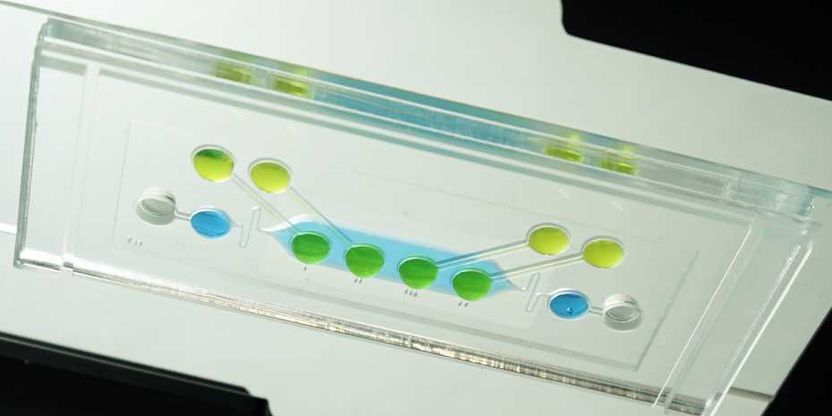
The chip hosting embryonic tissue in suspended drops of culture medium (in green), viewed from below. Placental cells are cultivated in the light blue area in the centre of the photograph.
ETH Zürich / Julia Boos
Drugs must be safe not just for the patients; in the case of pregnant patients, drugs must also be safe for the unborn children still in the womb. Therefore, at an early stage in the development of new medicines, candidate substances are tested in the Petri dish on embryonic stem cells from mouse cell lines. This is to avoid that an embryo-damaging effect would only be noticed at a later stage during tests with pregnant mice.
However, these cell culture tests are a highly simplified version of what takes place in the uterus. Researchers just add the test material to a culture of embryonic stem cells in a Petri dish, and can identify substances that have a direct adverse effect on embryonic cells. By contrast, in the body of a pregnant woman, active pharmaceutical ingredients may be modified by the mother’s metabolism and enter the embryo’s bloodstream via the placenta. Moreover, standard cell culture tests can’t detect substances that have indirect effects on the embryo, for example, in that they interfere with the functioning of the placenta or generate stress responses.
A chip with different cell types
Researchers in the Department of Biosystems Science and Engineering at ETH Zurich in Basel have now devised a laboratory test that incorporates the role of the placenta into embryotoxicity assessments. To do so, Julia Boos, a doctoral student in the group of ETH Professor Andreas Hierlemann, and her colleagues developed a new chip. This chip contains several compartments, all interconnected by miniature channels. On this chip, the scientists combined human placental cells taken from cell lines with microtissue spheroids derived from mouse embryonic stem cell lines, known as “embryoid bodies”, which reflect the early development of the embryo. Test substances first encounter a layer of placental cells, which they have to pass before reaching the embryonic cells, thereby reproducing the situation in utero.
Incidentally, these experiments do not produce viable embryos. The embryonic cells from cell lines only undergo the very first steps of embryonal development over a period of ten days.
Test detects indirect damage
To demonstrate the functioning of the new test, the researchers used microparticles that did not harm the embryoid bodies if they came into direct contact. With the new test, which also includes placental cells, however, the scientists observed a potential indirect adverse effect. Although the placental cells managed to hold the microparticles back, meaning the particles did not get through to the embryonic cells, the placental cells showed a detectable stress response.
Now the researchers would like to further develop their system with regard to more suitable plastic materials. It is also conceivable to use human stem cell lines, instead of mouse cells, to form embryoid bodies in the future. “There are significant differences between lab animals and humans, particularly in terms of embryonic development and the processes taking place in the placenta,” Boos says, continuing: “Of all the organs, the placenta is where differences between the species are most pronounced.”
The group aims at creating a new test that is also easy to use for the pharmaceutical industry. Being able to detect – and eliminate – substances that are harmful to the embryo at an early stage of drug development means that fewer substances will subsequently be tested on animals in in-vivo studies.
Original publication
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

New Method for Analysing Nanoporous Materials - Innovative method opens up new possibilities for materials science

A milestone on the pathway to Lab 4.0: A new standard for the smart lab - SPECTARIS presents the first industrial communication standard for laboratory and analytical devices

Fish and seafood - improved trace detection of life-threatening allergy sources - "AQUALLERG-ID": Researchers develop methods for detecting potential food allergens
PerkinElmer appoints President & Chief Operating Officer
ECHA calls for information to avoid unneccessary animal testing
Newly improved NIST reference material targets infant formula analysis

LAUDA appoints Dr. Ralf Hermann as the new General Manager Constant temperature equipment

A relative from the Tianyuan Cave - Ancient DNA has revealed that humans living some 40,000 years ago in the area near Beijing were likely related to many present-day Asians and Native Americans
Measurable for the first time: How bio molecules react to lack of space - Sensor demonstrates lack of space in living cells

Using AI to identify genetic perturbations from cell images - Newly founded start-up aims to use findings to treat previously incurable fibrosis




















































