How deadly parasites ‘glide’ into human cells
X-ray analysis reveals structure of molecular machinery of malaria and toxoplasmosis pathogens
An investigation at DESY's X-ray source PETRA III provides new insights into the molecular machinery by which certain parasites travel through the human organism. The study, led by Christian Löw from the Hamburg branch of the European Molecular Biology Laboratory EMBL, analyzed the so-called gliding movement of the malaria and toxoplasmosis parasites. The results, which the interdisciplinary team presents in the journal Communications Biology, can aid the search for new drugs against the pathogens.
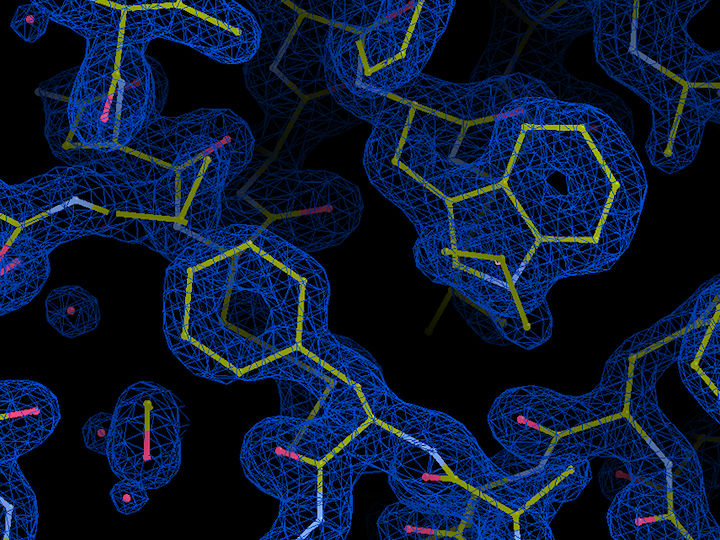
Molecular structure of essential light chain (ELC) protein in Plasmodium glideosome. Blue represents the electron density of the protein, with bonds between atoms indicated in yellow and water molecules indicated in red. The crystal structure at a resolution of 1.5 Ångström (0.15 millionths of a millimetre) was obtained at the EMBL beamlines at DESY’S X-ray source PETRA III.
EMBL, Samuel Pazicky
In biological terms, gliding refers to the type of movement during which a cell moves along a surface without changing its shape. This form of movement is unique to parasites from the phylum Apicomplexa, such as Plasmodium and Toxoplasma. Both parasites, which are transmitted by mosquitoes and cats, have an enormous impact on global heath. Plasmodium causes 228 million malaria infections and around 400 000 deaths per year. Toxoplasma, which infects even one third of the human population, can cause severe symptoms in some people, and is particularly dangerous during pregnancy.
Gliding enables the Apicomplexa parasites to enter and move between host cells. For example, upon entering the human body through a mosquito bite, Plasmodium glides through human skin before crossing into human blood vessels. This type of motion relies on actin and myosin, which are the same proteins that enable muscle movement in humans and other vertebrates. Myosin has a form of molecular ‘legs’ that ‘march’ along actin filaments and thereby create movement.
In Apicomplexa, myosin interacts with several other proteins, which together form a complex called the glideosome. The exact mechanism by which the glideosome works is not well understood, among other reasons because the molecular structure of most glideosome proteins are unknown. Yet understanding this mechanism could aid the development of drugs that prevent the assembly of the glideosome and thereby stop the progression of diseases such as malaria and toxoplasmosis.
Scientists at EMBL Hamburg analysed the molecular structure of essential light chains (ELCs), which are glideosome proteins that bind directly to myosin. It is known that they are necessary for gliding, but their exact structure and role were unknown until now. The researchers now obtained molecular structures of ELC bound to myosin A in Toxoplasma gondii and Plasmodium falciparum using X-ray crystallography at EMBL’s beamlines at PETRA III and nuclear magnetic resonance (NMR). Their study shows that ELCs work like ‘molecular stilts’: upon binding myosin A, the ELCs become rigid, and start to act as its lever arm. This stiffening lets myosin makes longer steps, which likely accelerates the parasite’s gliding movements.
The researchers also investigated the role of calcium, a presumed gliding regulator, in the interaction between ELCs and myosin A. Surprisingly, they discovered that calcium does not influence the structure of ELCs. It does, however, increase the stability of the ELC-myosin A complex. This unexpected result shows that the glideosome architecture still hides many unknowns.
The work is a result of interdisciplinary collaboration between structural biologists and parasitologists from EMBL and the Centre for Structural Systems Biology CSSB on the DESY campus in Hamburg, as well as scientists from the Bernhard Nocht Institute for Tropical Medicine in Hamburg, University of Hamburg and Martin-Luther-University Halle-Wittenberg.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!