Uncommon mineral discovered in Rembrandt painting
Maestro's Techniques
Rembrandt van Rijn’s paintings are renowned for their masterful representations of light and shadow and a characteristic plasticity generated by a technique called impasto. Now, scientists have analyzed impasto layers in some of Rembrandt’s paintings, and the study, which is published in the journal Angewandte Chemie, reveals that the impasto unexpectedly contains a very rare lead mineral called plumbonacrite. This finding suggests that Rembrandt used a unique paint recipe.
© Wiley-VCH
“Before the study, one of the only [pieces of] information about impastos was that they were achieved using lead white pigment,” says Victor Gonzalez at the Delft University of Technology, the Netherlands, who is a leading author of the study, which was conducted in collaboration with the Rijksmuseum, Amsterdam, the Netherlands, the Institut de Recherche de Chimie Paris, France, as well as French and Dutch Universities. “However, the precise recipe that Rembrandt used to achieve his impastos was not known,” he adds.
Lead white has been used in paintings since ancient times and, even today, its painterly properties still exceed those of its less toxic analogues. At the time Rembrandt created his artworks, lead white was produced by corrosion of metallic lead, resulting in the formation of a mixture of lead carbonates containing cerussite (lead carbonate, PbCO3) and hydrocerussite (Pb3(CO3)2·(OH)2), which is a shiny white, mixable, and fast-drying powder.
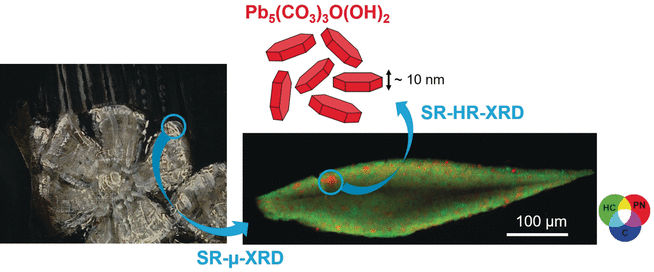
For their analysis, the scientists sampled tiny amounts of impasto paint layers in Rembrandt’s Portrait of Marten Soolmans of 1634, Susanna, 1634, and Bathseba, 1654. Using a combination of synchrotron X-ray diffraction at the European Synchrotron Radiation Facility in Grenoble, France, they identified hydrocerussite and cerussite, but surprisingly, they also found another lead mineral, plumbonacrite. “Plumbonacrite is extremely rare in historic paint layers,” explained the authors. “Its more notable occurrence was linked to degradation of the red lead (minimum) pigment in a Van Gogh painting,” they added.
In addition, the researchers detected plumbonacrite solely in the impasto layer, never in the layers below, and the other component of lead white, cerussite, was nearly absent. Why was the impasto chemical composition different from that of the underlayer although apparently the same pigments were used?
The scientists tried to find answers by following the chemistry of lead compounds. Plumbonacrite is only stable in alkaline (basic) environments. Under the acid production conditions of lead white, the mineral rapidly transforms into (hydro)cerussite. In contrast, a stable, highly basic lead mineral is litharge (PbO). Arguing that PbO was sometimes used as a binding additive at the time, the authors proposed that Rembrandt could have added PbO in the binder for his impasto. This would explain the composition of the white pigments. In a medium made alkaline by the litharge binder, lead carbonates would back-transform to plumbonacrite.
As a next step, the authors plan to investigate other Rembrandt paintings to check if the artist consistently used the same recipe. From the point of view of art history, it would also be important to see if other painters used the same technique.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!