FDA Clears the First Rapid Test to Detect Bacteria in Pooled Platelets
Experts to Re-examine Guidance for Platelet Testing
Fenwal, Inc. announced that the U.S. food and Drug Administration has cleared the Verax Platelet PGD(R) test as a quality control test to detect bacterial contamination in whole blood-derived, pooled platelets prior to transfusion. Fenwal is the exclusive global distributor of the test, which was developed by Verax Biomedical of Worcester, Mass.
Bacterial contamination in platelets is a serious threat to transfusion safety. More than 5 million platelet doses are transfused annually worldwide. Studies show that up to 1 in 2,000 doses may contain bacteria, which can cause a range of reactions, including death, especially in immune-compromised patients.
"Until now, there was no rapid test cleared by the FDA for detecting bacteria in whole-blood derived platelets," said Louis M. Katz, M.D., chair of international blood transfusion association AABB 's Task Force on Bacterial Contamination. "With such a test now available, we will reexamine the current Standards regarding 'methods to limit and to detect bacteria in all platelet components."
Most read news
Other news from the department research and development

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Malvern Instruments and Paraytec enter into development and technology licensing agreement

Blood samples from the zoo help predict diseases in humans
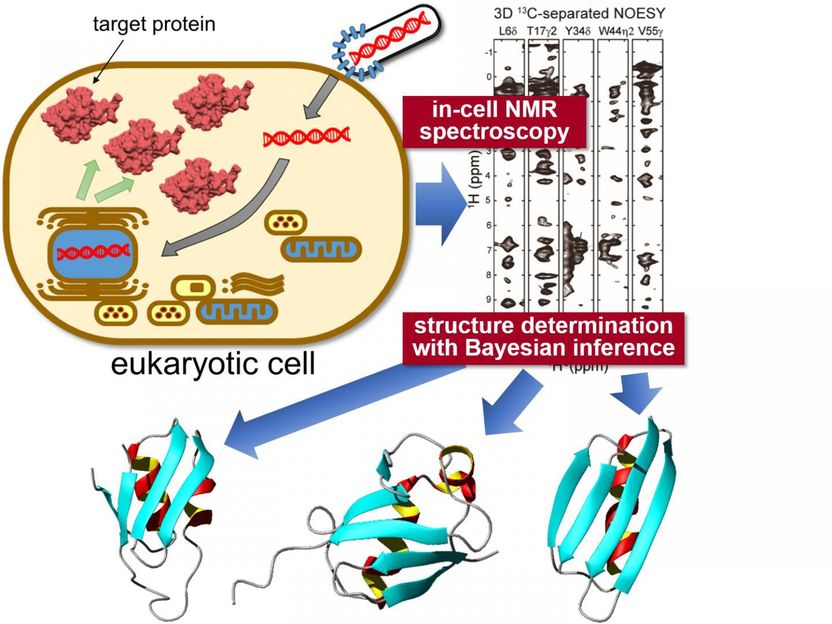
In-situ measurement of 3D protein structure inside living eukaryotic cells - Nuclear magnetic resonance measurement and state-of-the-art computational science reveal protein structures in higher eukaryotic cells
BioTek Introduces New General Manager in Germany

A Record Year for ZEISS - In fiscal year 2020/21, ZEISS achieves highest revenue yet in its 175-year history at 7.5 billion euros

Nanophoton Raman Microscopes | Raman microscopes | Bruker
Agilent Technologies Partners with China Environmental Centre to Detect Toxins in Water and Soil
Biotage AB joins forces with MultiSynTech GmbH and enters the peptide synthesis business































































