Epigenomics: Licensee ARUP Laboratories Launches Septin9 Colorectal Cancer Blood Test in the United States
Epigenomics AG reports that its licensee ARUP Laboratories, Salt Lake City, UT, U.S.A., today has announced the launched of a laboratory-developed test for the blood-based detection of colorectal cancer. The test is based on Epigenomics' proprietary Septin9 biomarker and DNA methylation technologies non-exclusively licensed to ARUP in August 2009.
According to ARUP the independently developed and validated Septin9 test identifies nine out of ten people with previously undetected colorectal cancer, including those with early stage disease. ARUP states that the test, which is now available to physicians and patients in the U.S., is not meant to replace colonoscopy but primarily aims at patients who cannot or will not undergo the established screening methods.
Most read news
Other news from the department research and development

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Bio-Techne to acquire Quad Technologies
BÜCHI acquires NIR-Online
Opto-electronic nose sniffs out toxic gases
ORNL scientists help explain graphene mystery
Altmann Analytik acquires lab supplier Dinkelberg analytics

New organ-on-chip pilot seeks to reduce animal testing in consumer health industry - Cooperation between Bayer, the start-ups esqLABS and Dynamic42, and Placenta Lab of Jena University Hospital

New method provides more precise information on types of leukaemia - Optical genome mapping could become a component of routine diagnostics
Oxford Nanopore Announces Licence Agreement with Harvard University for Graphene DNA sequencing

Waters and BD's Biosciences & Diagnostic Solutions Business to Combine - Creating a Life Science and Diagnostics Leader Focused on Regulated, High-Volume Testing

World's first toxicology testing strategy without animal testing adopted by OECD - Success for long-standing collaboration between BASF and Givaudan to develop and validate alternative methods
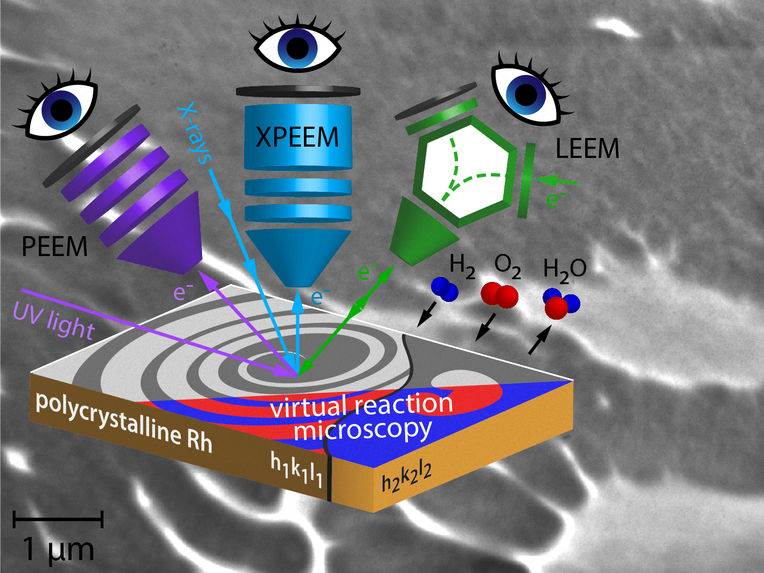
Three Eyes See More than Two - monitoring a catalytic reaction with three different microscopies under exactly the same conditions in real time - Information is obtained that none of the methods alone could reveal