How biomolecules react to UV light
Innovative experiment reveals ultrafast dynamics of proteins in their environment
A sophisticated experiment provides new insights into the ultrafast reaction of biomolecules when they are hit by energetic ultraviolet light in their natural environment. The team of DESY scientist Jochen Küpper recorded the light-induced processes taking place in a small complex of two molecules that served as model system for the interactions between proteins and surrounding solvent molecules, usually water. The experiment is an important step on the way to record a “molecular movie” of such chemical reactions, as the team reports in the journal Nature Communications. Küpper is DESY Lead Scientist for controlled molecule imaging.
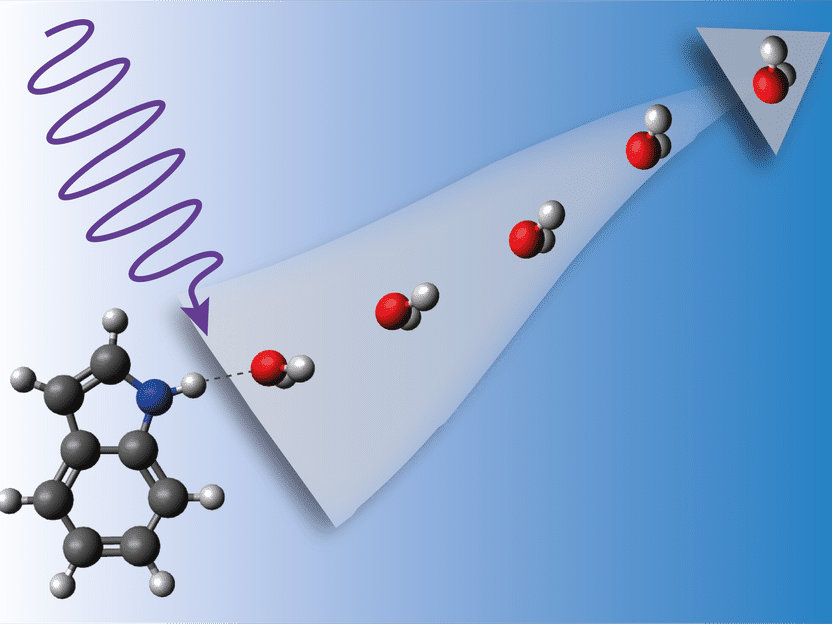
When the indole-water complex is hit by energetic UV light, it begins to vibrate violently until the water detaches.
Radboud-Universität, Jolijn Onvlee
“In our bodies, proteins generally occur in an aqueous environment, where the individual proteins are surrounded by water molecules,” explains main author Jolijn Onvlee, who now works as assistant professor at Radboud University in The Netherlands. The interactions between the protein and surrounding solvent (water) molecules affect how the proteins fold and thereby their function. “We specifically studied the UV-light-induced ultrafast dynamics of protein models in an aqueous environment in a bottom-up approach using a small aggregate of a so-called chromophore, a molecule that absorbs light and thereby gets excited, with a single water molecule attached,” reports Onvlee.
As a model system, the scientists chose the aromatic compound indole (C8H7N), which is the chromophore of the amino acid tryptophan and thus present in essentially all proteins. To investigate the interactions between proteins and their solvent surrounding, the scientists attached water to the indole molecules. However, indole can bind to several water molecules. To single out compounds of exactly one indole and one water molecule, the team used an electrostatic deflector that disperses the compounds in different directions depending on the number of water molecules attached.
The scientists shot pulses from a UV laser at the indole-water aggregates. These aggregates absorb the UV light, after which complex dynamics take place on the femtosecond (quadrillionths of a second) and picosecond (trillionths of a second) timescales. A second laser was used to record the resulting reaction products on an imaging detector. By changing the delay between the two lasers, snapshots of the ultrafast dynamics could be made at different stages, revealing the course of events. “When the UV light strikes the complex, excited state dynamics take place first, and after electronic relaxation the system starts vibrating vigorously,” explains Küpper. “The indole molecule is feeling totally hot.” Finally, it detaches from the water.
“Thanks to the combination of the electrostatic deflector and time-resolved imaging, we could now disentangle these ultrafast processes taking place in the complex,” says Küpper. “Ultimately, we would like to record an atomic-resolution ‘molecular movie’ in which we really see the path of the water leaving the indole molecule. The present research brings us an important step closer to reaching this goal.”
Original publication
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.