Alternative to high cost palladium catalysts?
Scientists in the USA have developed a ligand-free copper catalysed procedure for carbon-nitrogen bond formation.
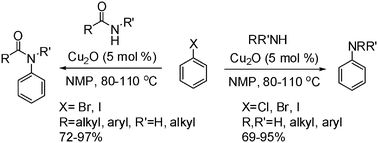
Christian Wolf and Hanhui Xu from Georgetown University, Washington, in the US, show that carbon-nitrogen bond formation can be achieved in high yield by a copper catalysed coupling procedure.
Wolf and Xu show that the amination of aryl chlorides, bromides and iodides with aromatic and aliphatic amines proceed efficiently using 5 mol% of Cu2O as the catalyst in N-methyl pyrrolidinone.
Significantly, the reaction does not require the use of activating ligands, and both primary and secondary amines, even amino acids and diphenylamine have been used in the reaction.
‘This procedure is generally useful, it utilises an inexpensive and readily available catalyst, and it provides aryl amines and amides in high yields,’ says Wolf.
Wolf hopes the copper catalyst will be useful in industry as an alternative to high cost palladium catalysts.
Original article: Wolf et. al.; "Copper catalyzed coupling of aryl chlorides, bromides and iodides with amines and amides"; Chem. Commun., 2009
Most read news
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.