Scientists unveil world’s first molecular-level analysis of Omicron spike protein
Findings shed light on factors behind Omicron’s increased transmissibility
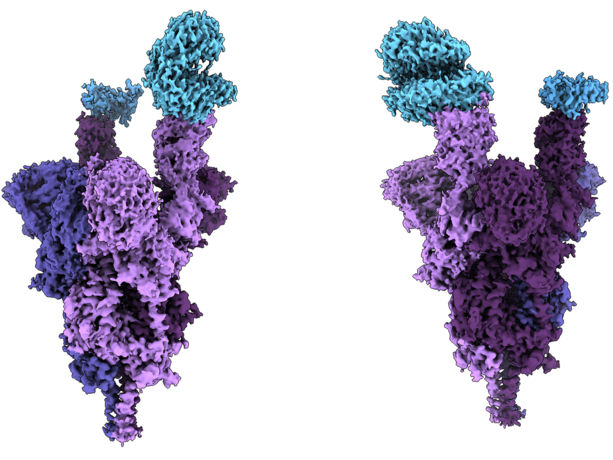
Researchers at UBC’s faculty of medicine have conducted the world’s first molecular-level structural analysis of the Omicron variant spike protein. The findings were published in Science.
UBC Faculty of Medicine
The analysis – done at near atomic resolution using cryo-electron microscopy – reveals how the heavily mutated Omicron variant attaches to and infects human cells.
“Understanding the molecular structure of the viral spike protein is important as it will allow us to develop more effective treatments against Omicron and related variants in the future,” said lead author Dr. Sriram Subramaniam (he/him), professor in UBC’s department of biochemistry and molecular biology. “By analyzing the mechanisms by which the virus infects human cells, we can develop better treatments that disrupt that process and neutralize the virus.”
The spike protein, which is located on the outside of a coronavirus, enables SARS-CoV-2 to enter human cells. The Omicron variant has an unprecedented 37 mutations on its spike protein – three to five times more than previous variants.
The structural analysis revealed that several mutations (R493, S496 and R498) create new salt bridges and hydrogen bonds between the spike protein and the human cell receptor known as ACE2. The researchers concluded that these new bonds appear to increase binding affinity – how strongly the virus attaches to human cells – while other mutations (K417N) decrease the strength of this bond.
“Overall, the findings show that Omicron has greater binding affinity than the original virus, with levels more comparable to what we see with the Delta variant,” said Dr. Subramaniam. “It is remarkable that the Omicron variant evolved to retain its ability to bind with human cells despite such extensive mutations.”
The researchers conducted further experiments showing that the Omicron spike protein exhibits increased antibody evasion. In contrast to previous variants, Omicron showed measurable evasion from all six monoclonal antibodies tested, with complete escape from five. The variant also displayed increased evasion of antibodies collected from vaccinated individuals and unvaccinated COVID-19 patients.
“Notably, Omicron was less evasive of the immunity created by vaccines, compared to immunity from natural infection in unvaccinated patients. This suggests that vaccination remains our best defence,” said Dr. Subramaniam.
Based on the observed increase in binding affinity and antibody evasion, the researchers say that the spike protein mutations are likely contributing factors to the increased transmissibility of the Omicron variant.
Next, Dr. Subramaniam says his research team will leverage this knowledge to support the development of more effective treatments.
“An important focus for our team is to better understand the binding of neutralizing antibodies and treatments that will be effective across the entire range of variants, and how those can be used to develop variant-resistant treatments.”
Original publication
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.