Tin-100, a doubly magic nucleus
A few minutes after the Big Bang the universe contained no other elements than hydrogen and helium. Physicists of the Technische Universitaet Muenchen (TUM), the Cluster of Excellence “Universe” and the Helmholtz Center for Heavy Ion Research (GSI) have now succeeded in producing tin-100, a very instable yet important element for understanding the formation of heavier elements.
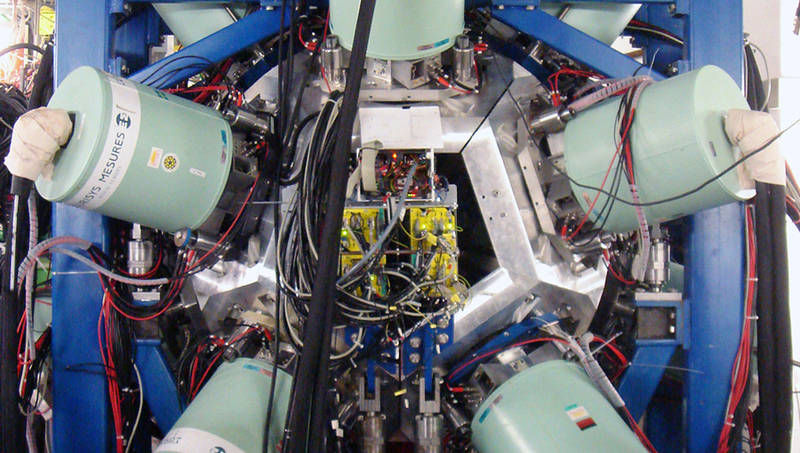
A view of the experiment at the GSI from a perspective against the beam direction. The fragments are stopped at the center of a “hedgehog” of 105 liquid nitrogen-cooled gamma ray detectors, where the precise time point of the beta decay and the released decay energy are measured.
Thomas Faestermann / TUM
A few minutes after the Big Bang the universe contained no other elements than hydrogen and helium. Physicists of the Technische Universitaet Muenchen (TUM), the Cluster of Excellence “Universe” and the Helmholtz Center for Heavy Ion Research (GSI) have now succeeded in producing tin-100, a very instable yet important element for understanding the formation of heavier elements. The researchers report on their results in the current edition of the scientific journal Nature.
Stable tin, as we know it, comprises 112 nuclear particles – 50 protons and 62 neutrons. The neutrons act as a kind of buffer between the electrically repelling protons and prevent normal tin from decaying. According to the shell model of nuclear physics, 50 is a “magic number” that gives rise to special properties. Tin-100, with 50 protons and 50 neutrons, is “doubly magic,” making it particularly interesting for nuclear physicists.
Shooting xenon-124 ions at a sheet of beryllium, the international team headed by physicists from the TU Muenchen, the Cluster of Excellence Origin and Structure of the Universe and the GSI in Darmstadt succeeded in creating tin-100 and analyzing its subsequent decay. Using specially developed particle detectors, they were able to measure the half-life and decay energy of tin-100 and its decay products. Their experiments confirmed that tin-100 has the fastest beta decay of all atomic nuclei, as previously predicted by theoretical physicists.
A repeat of the experiment is slated for the near future at the RIKEN research center in Japan. The beam intensity at RIKEN is higher in the mean time, allowing even more precise measurements. The aim of the research work is to improve the understanding of processes in the formation of heavy elements during explosions on the surface of compact stars. In addition, the researchers hope to draw conclusions on the neutrino mass from the measurements.
This work was supported by the BMBF, by the GSI, by the DFG-Cluster of Excellence Origin and Structure of the Universe, by the EC within the FP6 through I3-EURONS and by the Swedish Research Council.
Original publication
Other news from the department science

Get the analytics and lab tech industry in your inbox
From now on, don't miss a thing: Our newsletter for analytics and lab technology brings you up to date every Tuesday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.