LABS/Q LIMS
LABS/Q LIMS - The professional standard solution for all laboratories
GUS LAB GmbH
Configurable standard LIMS
Can be used across locations and in all organizational sizes
Masks and database fields individually adaptable and expandable


Cross-Industry Laboratory Application, Which Has Been Used Successfully for More than 30 Years
With LABS/Q ® you can manage all your laboratory data in one application and you only need to enter the required master data. If you already have data in another system, we migrate these data via an an automated process. Advantages of LABS/S LIMS: - Lower costs in the implementation phase - Shorter project times during implementation - The vendor qualifies (tests) the functionality - Easy upgradeability of the product - Lower risk for the whole project

1
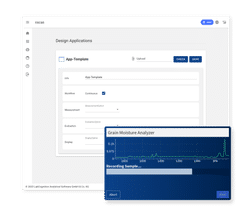
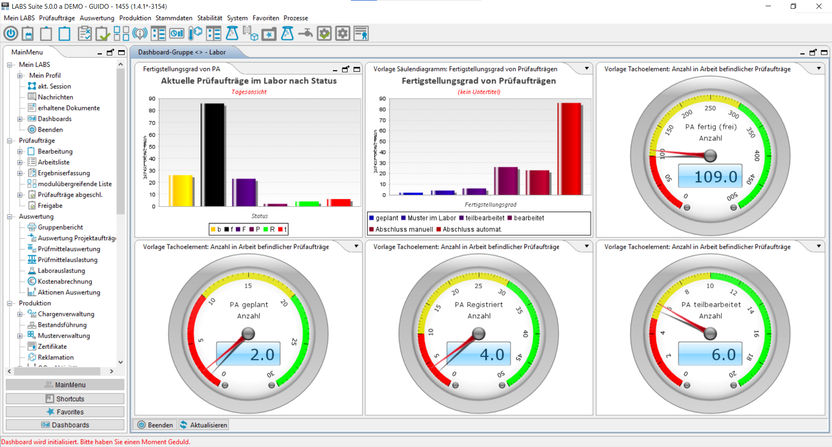
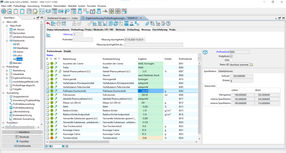
LIMS Dashboard

2
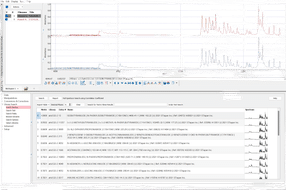
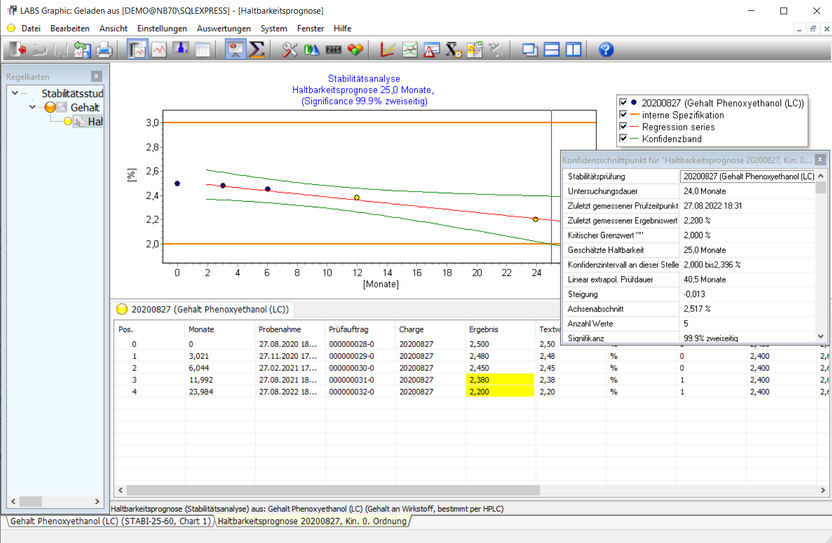
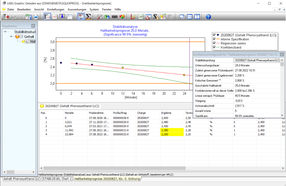
Example of a stability analysis, the graphical and statistical analysis functions are shown. The LABSGraphic part of LABS/Q® offers various graphical and statistical analysis functions

3
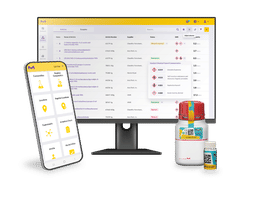
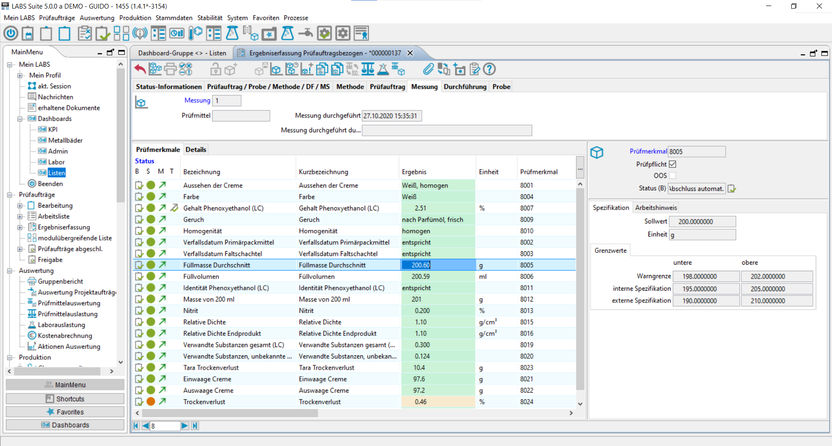
The test- and measuring equipment monitoring is the backbone of the AQA in the lab and replaces the device management books as well as it helps keeping track of the standard and control samples

4
Experience this product live at the trade fair
Request information about LABS/Q LIMS now

LIMS software: LABS/Q LIMS
LABS/Q LIMS - The professional standard solution for all laboratories
Find more LIMS software and related products
Find LABS/Q LIMS and related products in the theme worlds
analytica preview 2024
Here, innovative exhibitors present their trade fair innovations, premieres and product innovations for analytica 2024.

analytica preview 2024
Here, innovative exhibitors present their trade fair innovations, premieres and product innovations for analytica 2024.
© 1997-2024 LUMITOS AG, All rights reserved