Peptide complex formed in the brain is responsible for Alzheimer's disease
Members of the Faculty of Fundamental medicine at the Lomonosov Moscow State University have determined the structure of a peptide complex, formed in the brain at the early stages of Alzheimer's disease progression. The research results will contribute to the rational design of compounds, capable to block disease progress.
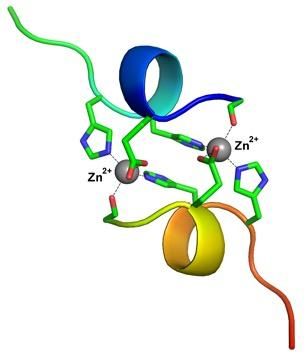
The structure of the complex of β-amyloid fragment -- the product of Taiwanese mutation, with zinc ions.
Vladimir Polshakov
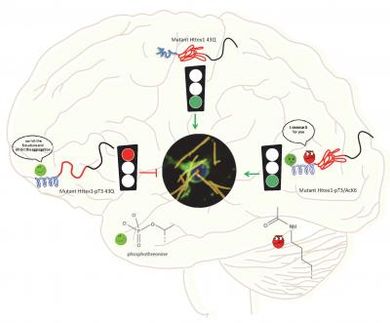
Alzheimer's disease is associated with the aggregation of amyloid-β peptide in the human brain. The scientific team from the Lomonosov Moscow State University under the leadership of Vladimir Polshakov, Doctor of Chemistry, has studied molecular mechanisms of β-amyloid aggregation among the carriers of pathogenic familial Taiwanese mutation, described the structure of emerging complexes and established the role of zinc ions (Zn2+) in their formation.
β-Amyloid is a small peptide, implementing important physiological functions, for instance, protecting the brain from potential pathogenic agents. Normally, after fulfilling its functions the peptide is cleaved by proteases and utilized. However, in some cases molecules of this peptide start binding with each other, forming complexes toxic for neurons. Processes of β-amyloid aggregation into such complexes are initiated by transition metal ions.
During several years the scientists from the Lomonosov Moscow State University in cooperation with their colleagues from the Engelhardt Institute of Molecular Biology have been studied the molecular mechanism of zinc-mediated aggregation of β-amyloid peptide. They have chosen the peptide carrying the Taiwanese mutation as a model. Alzheimer's disease inevitably progresses among the carriers of such mutations at a relatively young age. The researchers have revealed that the structure of the β-amyloid complexes, consisting of mutant peptides, turns out to be tighter and more stable compared to the complexes from native peptides. Zinc ions (Zn2+) play the key role in their formation.
Vladimir Polshakov comments: "We were surprised to see that interaction between a fragment of this peptide and zinc ions has led to formation of a stable complex, where two peptide chains are tightly fastened by two zinc ions. Similar binuclear structures haven't been described in the literature yet. It's important to notice that interaction between metal ions and β;-amyloid peptides usually leads to the variety of peptide chain conformations. The emerging complexes are sort of "breathing", passing from one conformation to another. But in case of the peptide - product of Taiwanese mutation we observed the only single conformation, which allowed us to determine its structure with high accuracy and precision, using the methods of nuclear magnetic resonance spectroscopy."
The obtained results will help to design compounds, capable to block zinc-mediated β-amyloid aggregation, which, in its turn, would stop the progress of Alzheimer's disease at early stages. Vladimir Polshakov shares with the research perspectives: "Using the information on the molecular mechanism, revealed in this project, which initiates pathogenic aggregation of β-amyloid peptide, our colleagues from the Engelhardt Institute of Molecular Biology have already taken out patents for two compounds, capable of terminating β-amyloid aggregation. Studies of properties of these compounds on animal models have proved that they are able to reduce by several times the risk of progress of a disease, analogous to Alzheimer's disease in human."
The members of the Laboratory of Magnetic Tomography and Spectroscopy, working at the Faculty of Fundamental Medicine, the Lomonosov Moscow State University have conducted the research in tight collaboration with the scientific team from the Engelhardt Institute of Molecular Biology (Russian Academy of Sciences) under the guidance of Academician Alexander Makarov.
Original publication
Vladimir I. Polshakov, Alexey B. Mantsyzov, Sergey A. Kozin, Alexei A. Adzhubei, Sergey S. Zhokhov, Wouter van Beek, Alexandra A. Kulikova, Maria I. Indeykina, Vladimir A. Mitkevich, Alexander A. Makarov; "A Binuclear Zinc Interaction Fold Discovered in the Homodimer of Alzheimer's Amyloid‐β Fragment with Taiwanese Mutation D7H"; Angew. Chem. Int. Ed.; 2017
Original publication
Vladimir I. Polshakov, Alexey B. Mantsyzov, Sergey A. Kozin, Alexei A. Adzhubei, Sergey S. Zhokhov, Wouter van Beek, Alexandra A. Kulikova, Maria I. Indeykina, Vladimir A. Mitkevich, Alexander A. Makarov; "A Binuclear Zinc Interaction Fold Discovered in the Homodimer of Alzheimer's Amyloid‐β Fragment with Taiwanese Mutation D7H"; Angew. Chem. Int. Ed.; 2017
Topics
Organizations
Other news from the department science

Get the analytics and lab tech industry in your inbox
From now on, don't miss a thing: Our newsletter for analytics and lab technology brings you up to date every Tuesday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.