Image-tracking technology helps scientists study nature v. nurture in neural stem cells
One of the longstanding debates in science, that has, perhaps unsurprisingly, permeated into the field of stem cell research, is the question of nature versus nurture influencing development. Science on stem cells thus far, has suggested that, as one side of the existential debate holds: their fate is not predestined. But new research from the Neural Stem Cell Institute and Drexel University suggests that the cells' tabula might not be as rasa as we have been led to believe.
Drexel University
Researchers present voluminous video evidence to support their discovery of distinct, intrinsic differences among stem cells found in the brains of mice. Through time-lapse imaging of cells cultured in identical in vitro environments, researchers were able to identify differences in growth, movement and differentiation that exist between stem cells found in two different areas of the brain.
Neuro- Nature v. Nurture
The cerebral cortex is the control center of the brain, it is divided into areas with dedicated functions. The posterior or back of the cortex integrates visual stimuli from the eye and determines our responses by sending signals to the anterior. The visual cortex and the motor cortex are both layered structures, each containing millions of neurons networked into intricate circuits. During development as embryos, each of these parts of the cortex is created from neural stem and progenitor cells.
A key unanswered question in the study of neuroscience is whether the visual and motor cortex areas are produced by neural stem cells that are themselves unique and patterned to produce each area by cell-intrinsic programs, or whether the progenitor cells are essentially the same, but rely on environmental signals to carve out the unique cells and connections in each final cortex area. Understanding the mechanisms controlling stem cell production is not just important for understanding human development, but also for learning how to use stem cells for regenerative medicine and for formulating new cancer therapies.
To answer that question, Sally Temple, PhD, and her research group at the Neural Stem Cell Institute studied single neural stem cells from the anterior and posterior embryonic mouse cerebral cortex. They provided the cells with an identical environment in tissue culture, then made time-lapse movies of how they divided and differentiated into cortical cells.
"If the answer to the age-old question was, in fact, 'nurture' then these cells would behave the same, given the identical environments," Temple said. "But if the answer was 'nature' then they would behave differently. And this is actually what we found--anterior and posterior stem cells are intrinsically quite different from each other."
The anterior stem cells, which form the motor cortex, were observed to divide more slowly, and produce smaller clones in comparison to the stem cells derived from the posterior--which grow to form the visual cortex. This means that these cortical progenitor cells are different and these differences could help explain how areas of the cortex develop into specialized structures.
Tracking Cells in Time-Lapse
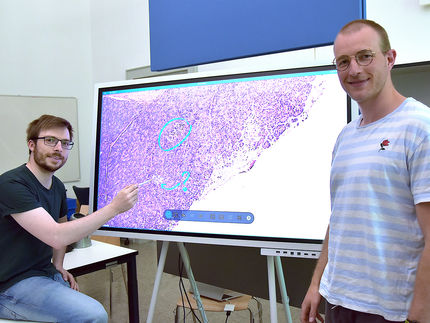
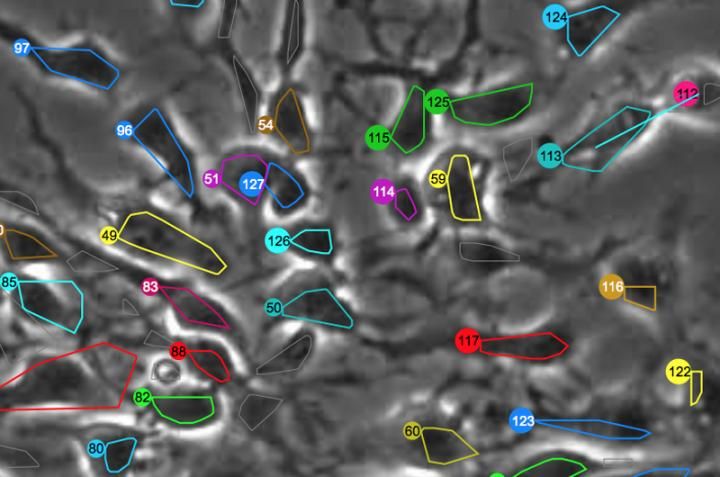
This discovery was made possible by a powerful set of biological tracking programs, developed in the Computational Image Sequence Analysis Lab led by Andrew Cohen, PhD, an associate professor in Drexel's College of Engineering, that automated the process of analyzing tens of thousands of time-lapse images. The programs, called LEVER and CloneView, can track the growth, movement and proliferation of cells and use this information to visually enhance the time-lapse movies and produce an entire family tree for each cell.
A Human Touch
Displayed in CloneView, a time-lapse movie of a single stem cell is overlaid by a uniquely colored outline of its inner boundary. As it grows and eventually divides, new cells are delineated with a different color. Each of these colors corresponds to the cell's node on the lineage tree, which is displayed in a separate frame next to the movie. This gives Cohen's research assistants a quick way to check the video to make sure LEVER correctly identified the cells and their progeny.
Once checked by human eyes, the images are run through the program again for more precise delineation of the cells--this process is especially arduous when tracking stem cells because they often have long arm-like appendages called processes that can look like a separate cell.
"Human validation is a very important part of our process. We are still the most accurate judge of when cells have divided," Cohen said. "By running the time-lapse images through the program we get an output video that's easy to visually verify. As these corrections are made, the program can spot related errors and automatically fix those. The key is to minimize the amount of effort required from the humans."