Zero in on ozone with fluorescent solution that detects harmful molecule in air and body
Personal ozone detectors and biomedical indicators could result from a chemical probe that glows bright green when exposed to ozone
Researchers at the University of Pittsburgh have developed a fluorescent substance that glows bright green when exposed to even minute amounts of ozone in the air and in biological samples such as human lung cells. A molecule consisting of three oxygen atoms, ozone is at once a harmful pollutant and lung irritant, and a possible natural weapon that certain research suggests the human body employs against infections.
The Pitt team reports in the current edition of Nature Chemistry that their simple and fast-acting detector can function as a consumer device to measure surrounding ozone, or as a laboratory tool that could provide insight into ozone's effect on the human body and its debated role in the human immune system. The probe differs from existing ozone-detection methods in that it's sensitive only to ozone, the researchers write. Current indicators can register a false-positive in response to humidity, other reactive oxygen species, and atmospheric compounds such as lead, palladium, and platinum.
"As you inhale air, you inhale ozone, and it is not known how deeply it penetrates the lung or its effect on the body," said Kazunori Koide, a chemistry professor in Pitt's School of Arts and Sciences, who is the paper's corresponding author. "Patients with respiratory diseases who are more sensitive to ozone may be able to monitor their exposure, as should employees in industrial and laboratory jobs that include regular ozone exposure. Our method is quick, so people will know they've exceeded safe levels before they suffer the symptoms, and it's highly specific to ozone, so it will prevent having false data."
Koide worked with researchers in the Department of Environmental and Occupational Health in Pitt's Graduate School of Public Health: professor George Leikauf, professor and department chair Bruce Pitt, and assistant professor Claudette St Croix. The project also included Pitt postdoctoral student Shin Ando and graduate student and lead author Amanda Garner, both in the Department of Chemistry.
The team's detection method consists of a small molecule-based probe added to regular distilled water. Ozone reacts with the probe through a process called ozonolysis, creating the organic compound aldehyde. The aldehyde undergoes an additional reaction known as beta elimination to produce a substance that glows bright green - or Pittsburgh Green, as the researchers termed it - under an ultraviolet (UV) lamp or microscope. The Pitt team reported that the solution began to glow within 30 minutes of coming into contact with ozone.
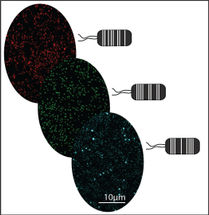
As an indoor and outdoor pollutant, ozone can irritate the lungs, particularly in people with asthma, bronchitis, or cystic fibrosis. It is generated by certain electronic devices and also created from the reaction of high concentrations of pollution, such as car exhaust and UV radiation from the sun. Koide and his colleagues sought to create an easy method for people to test the ozone-level of their immediate indoor and outdoor environment; they suggest in their paper that people could wear a badge containing the probe. For the indoor experiment, paper strips coated with the solution were left for eight hours in an unventilated office with two photocopiers and two laser printers, devices that are known to generate ozone. When exposed to UV light, the strips revealed concentrations of ozone captured from within the room. To test the probe outdoors, the Pitt scientists placed the solution at four high-traffic areas in Pittsburgh for eight hours on a sunny day (but out of direct sunlight) and successfully detected ozone.
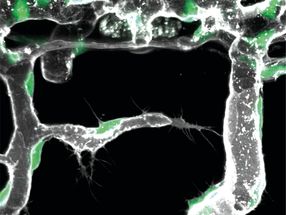
The probe also was tested on human lung fluid and blood serum to determine its biomedical potential. The samples were exposed to ozone and glowed under a laser light, showing that the probe could work in biological samples. The researchers went further and exposed human lung cells treated with the probe to ozone-rich air for five minutes. With a microscope, they observed the fluorescent glow expand within the cell, illustrating that ozone indeed penetrated the cell membrane.
The probe's successful use in biological samples could help unravel certain medical questions regarding ozone. The researchers cite a back-and-forth of research published in various journals such as Science and the Proceedings of the National Academy of Science that claim and dispute whether white blood cells emit ozone to fight inflammation and bacterial infections. So far, though, the methods used to detect ozone have been questioned based on their sensitivity to other reactive oxygen species, a problem the Pitt probe seems to not have.
Another issue concerns ozone's penetration of the body and its subsequent effect. The Pitt researchers note that other scientists have observed ozone's effect on cell samples, but that other findings suggest ozone is too short-lived to affect cells in the body. They demonstrated that their probe can track ozone as it moves throughout a cell sample and possibly help scientists gain insight into the molecule's activity within cells.